Pioneering Development of Novel Therapy
Cardiopulmonary disease robs people of their ability to be active, to perform the simplest of tasks, to engage with their families and loved ones… to live. Driven by the opportunity to give people living with cardiopulmonary disease greater control over their health, Aerovate is pioneering the development of novel therapies.
Our first program is AV-101, an investigational therapy targeting the hyperproliferation of cells in the pulmonary vasculature that underlies pulmonary arterial hypertension (PAH). By targeting the proliferation and accumulation of cells in the arteries of the lungs, AV-101 has the potential to provide meaningful improvements for patients beyond the capabilities of currently approved therapies.
Addressing Drivers of PAH
PAH is a disease that disproportionately affects women (65-80 percent), and often strikes in the prime of life, with the average age of those affected now being around 50. People with PAH experience a narrowing and blockage of the blood vessels in the lungs resulting in an increased resistance to blood flow into the lungs from the right side of the heart. This forces the heart to work harder to meet this abnormal demand, and over time the heart weakens and ultimately may fail.
Existing therapies, known as vasodilators, while effective at providing symptomatic relief, fail to treat the underlying cellular proliferation causing disease progression.
Aerovate wants to address this significant gap in therapy, with our targeted antiproliferative, AV-101, an investigational, dry powder formulation of imatinib for inhalation.
Imatinib is an antiproliferative agent that selectively targets the kinase signaling that has been implicated in causing aberrant cell growth in the pulmonary vasculature, while not interfering with signals needed for healthy cellular function.
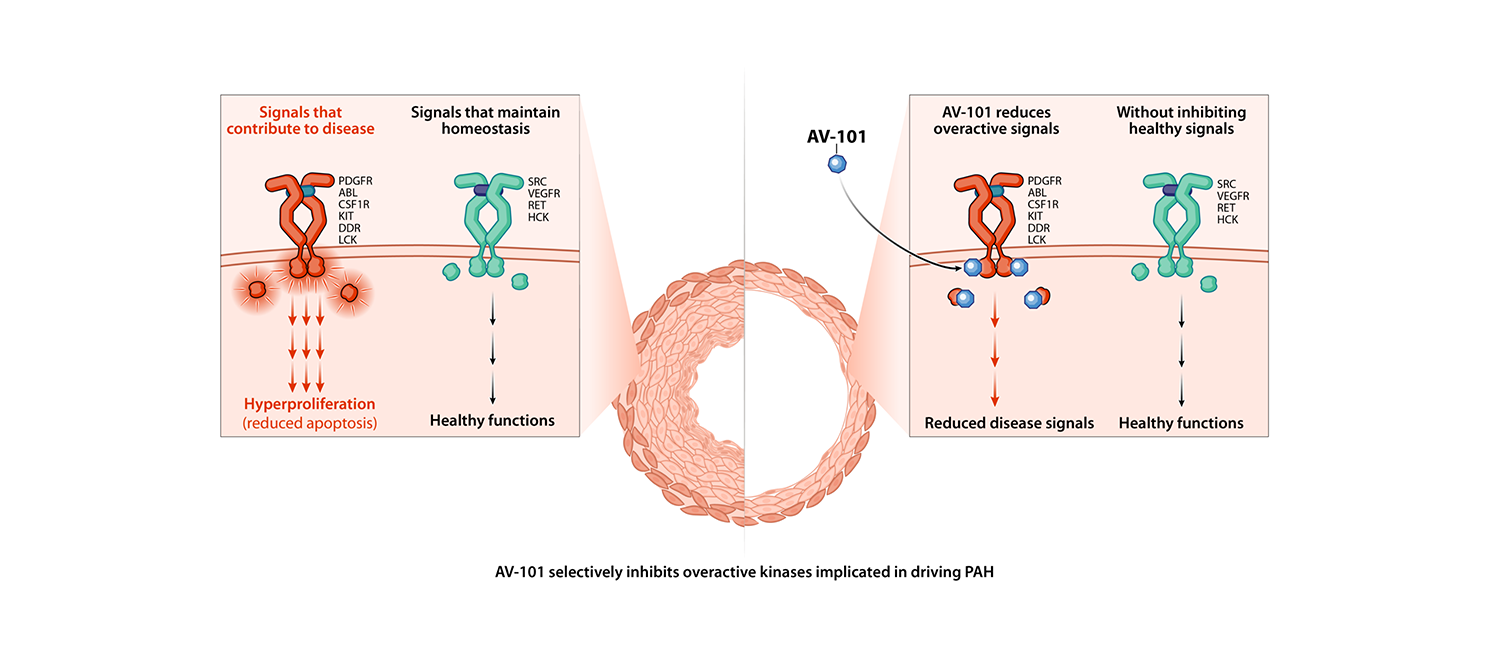
Imatinib is the first antiproliferative to have demonstrated clinical efficacy in phase 2 and phase 3 trials of PAH when administered orally. However, systemic side effects limited its therapeutic use, and it was not approved for the treatment of PAH.
AV-101 is a novel, dry powdered form of imatinib for inhalation that is designed to deliver antiproliferative therapy directly to the lungs, allowing more of the drug to access the diseased tissues directly while simultaneously reducing systemic exposure.

AV-101 has been designed to deliver high concentrations of imatinib throughout the airways where it can permeate throughout the lung and immediately reach the surrounding tissue and blood vessels — efficiently delivering AV-101 straight to diseased blood vessels.

By targeting hyperproliferation in the pulmonary vasculature and limiting systemic exposure, AV-101 has the potential to make a meaningful difference for the PAH community.
AV-101 in the Clinic
Although oral imatinib has demonstrated clinical efficacy in Phase 2 and Phase 3 trials in PAH patients, the systemic side effects limited the oral formulation’s therapeutic use, and it was not approved for the treatment of PAH. Dosed specifically for PAH, AV-101 is a novel dry powder form of imatinib for inhalation which is designed to deliver antiproliferative therapy directly to the lungs, allowing more of the drug to access the diseased tissues while simultaneously reducing systemic exposure. By limiting systemic side effects and targeting hyperproliferation in the pulmonary vasculature, AV-101 has the potential to make a meaningful difference for the PAH community.
In a recent Phase 1 clinical trial, Aerovate demonstrated AV-101 was generally well-tolerated by healthy volunteers with no serious adverse events reported.


