Pulmonary Vascular Research Institute Engage PVRI2021 Clinical abstract
The aerosol delivery of tyrosine kinase inhibitors (TKI) may improve risk benefit ratio in PAH but the overall molecular TKI fingerprint is important in determining clinical efficacy and safety
PVRI Engage PVRI2021 Video abstract
PVRI Engage. 2021 Jan 12; Pulmonary Vascular Research Institute PVRI2021 Clinical Abstracts (online).
Authors: Benjamin T. Dake, PhD & Hunter Gillies, MD
Aerovate Therapeutics
Pulmonary vasculopathy in PAH has similar pathogenic mechanisms to cancer (Cool 2020). Growth factors are instrumental in the vascular remodeling process via kinase dependent signaling through PDGFR, EGFR, VEGFR and IGF amongst others that appear to be important in PAH pathogenesis. Targeting these kinases with different small molecule inhibitors has been successful in oncology but use of the same drugs has resulted in discordant outcomes in PAH patients.
Imatinib demonstrated efficacy in the phase 3 IMPRES trial, but was hampered by systemic intolerability (Hoeper 2013). A similar kinase inhibitor nilotinib did not progress beyond phase 2 in PAH (NCT01179737), nintedanib did not show benefit in a small compassionate use trial (Richter 2018) despite positive preclinical data (Rol 2019, Tsutsumi 2019) and dasatinib is known to induce PAH in oncology patients (Shaw 2015). Reproducing the differential clinical effects of these drugs in toxicology studies and rodent models of PAH has been difficult (Pullamsetti 2012, Guignabert 2016, Baumgart 2017).
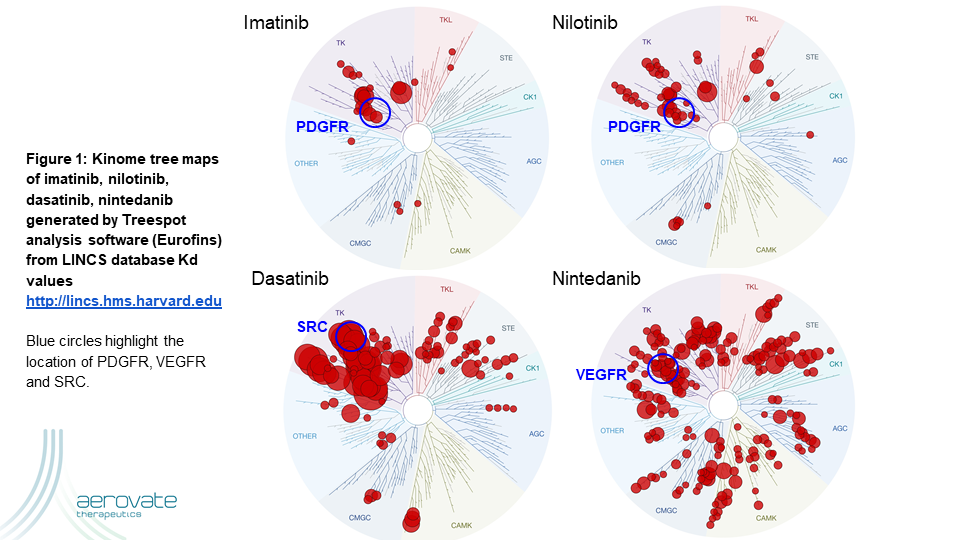
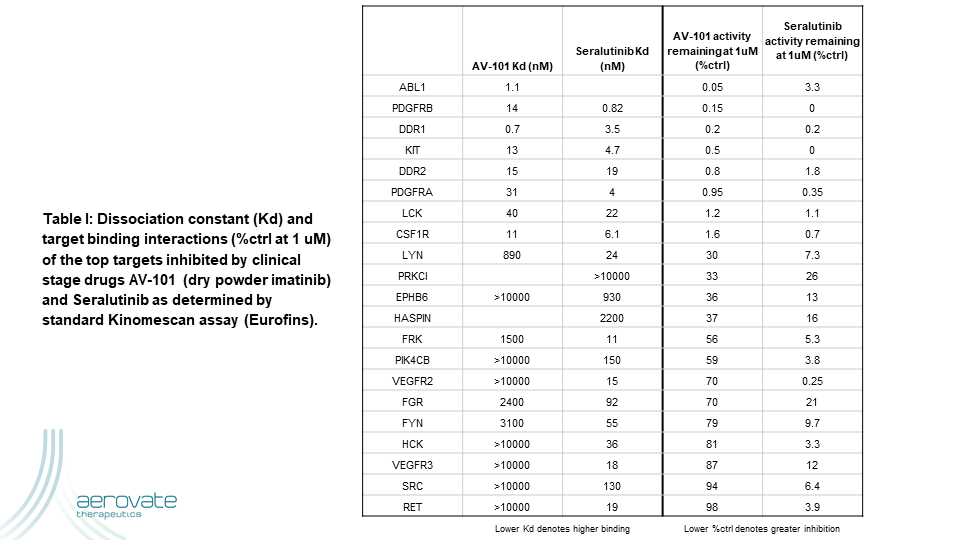
A novel clinical strategy to overcome the intolerability of TKIs in PAH is to focus the drug more directly where it is needed via aerosol administration. The higher local drug exposure in the diseased tissues could potentially result in reproducing or increasing efficacy from the oral route of administration while reducing systemic adverse effects. Two clinical stage kinase inhibitors pursuing this strategy via dry powder aerosols are AV-101 (dry powder imatinib) and seralutinib. Table I shows the top kinases inhibited by these two drugs using the standard Kinomescan assay (Eurofins).
There are multiple lines of evidence showing the importance of PDGFR signaling in animal models and in human PAH. However, the other targets of these drugs are not as well explored. These kinase inhibitors all target a conserved ATP-binding domain and thus it is well established that each molecule has its own fingerprint of targets and potencies. Figure 1 illustrates that Imatinib is the most selective kinase inhibitor targeting ABL, PDGFR, DDR, KIT and CSF1R at physiologic concentrations while not hitting SRC or VEGFR as dasatinib and nintedanib do respectively.
Conclusion: All of these drugs hit PDGFR but the clinical outcome is very different. Given the wealth of published data on PDGFR in PAH, it is reasonable to suggest that PDGFR inhibition is one component necessary for clinical success in PAH. Ultimately, the balance of inhibition with the other kinases determines the overall efficacy and safety in the clinic.
References:
Cool CD, Kuebler WM, Bogaard HJ, Spiekerkoetter E, Nicolls MR, Voelkel NF. The hallmarks of severe
pulmonary arterial hypertension: the cancer hypothesis-ten years later. Am J Physiol Lung Cell Mol Physiol. 2020
Jun 1;318(6):L1115-L1130. doi: 10.1152/ajplung.00476.2019. Epub 2020 Feb 5. PMID: 32023082.
Hoeper MM, Barst RJ, Bourge RC, Feldman J, Frost AE, Galié N, Gómez-Sánchez MA, Grimminger F, Grünig E,
Hassoun PM, Morrell NW, Peacock AJ, Satoh T, Simonneau G, Tapson VF, Torres F, Lawrence D, Quinn DA,
Ghofrani HA. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized
IMPRES study. Circulation. 2013 Mar 12;127(10):1128-38. doi: 10.1161/CIRCULATIONAHA.112.000765. Epub
2013 Feb 12. PMID: 23403476.
Richter MJ, Ewert J, Grimminger F, Ghofrani HA, Kojonazarov B, Petrovic A, Seeger W, Schermuly RT, Tello K,
Gall H. Nintedanib in Severe Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2018 Sep
15;198(6):808-810. doi: 10.1164/rccm.201801-0195LE. PMID: 29763335.
Rol N, de Raaf MA, Sun XQ, Kuiper VP, da Silva Gonçalves Bos D, Happé C, Kurakula K, Dickhoff C, Thuillet R,
Tu L, Guignabert C, Schalij I, Lodder K, Pan X, Herrmann FE, van Nieuw Amerongen GP, Koolwijk P,
Vonk-Noordegraaf A, de Man FS, Wollin L, Goumans MJ, Szulcek R, Bogaard HJ. Nintedanib improves cardiac
fibrosis but leaves pulmonary vascular remodelling unaltered in experimental pulmonary hypertension. Cardiovasc Res. 2019 Feb 1;115(2):432-439. doi: 10.1093/cvr/cvy186. PMID: 30032282.
Tsutsumi T, Nagaoka T, Yoshida T, Wang L, Kuriyama S, Suzuki Y, Nagata Y, Harada N, Kodama Y, Takahashi F,
Morio Y, Takahashi K. Nintedanib ameliorates experimental pulmonary arterial hypertension via inhibition of
endothelial mesenchymal transition and smooth muscle cell proliferation. PLoS One. 2019 Jul 24;14(7):e0214697.
doi: 10.1371/journal.pone.0214697. PMID: 31339889; PMCID: PMC6656344.
Shah NP, Wallis N, Farber HW, Mauro MJ, Wolf RA, Mattei D, Guha M, Rea D, Peacock A. Clinical features of
pulmonary arterial hypertension in patients receiving dasatinib. Am J Hematol. 2015 Nov;90(11):1060-4. doi:
10.1002/ajh.24174. Epub 2015 Oct 12. PMID: 26284693.
Pullamsetti SS, Berghausen EM, Dabral S, Tretyn A, Butrous E, Savai R, Butrous G, Dahal BK, Brandes RP,
Ghofrani HA, Weissmann N, Grimminger F, Seeger W, Rosenkranz S, Schermuly RT. Role of Src tyrosine kinases
in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2012 Jun;32(6):1354-65. doi:
10.1161/ATVBAHA.112.248500. Epub 2012 Apr 19. PMID: 22516066.
Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, Sattler C, Le Hiress M, Tamura Y, Jutant EM,
Chaumais MC, Bouchet S, Manéglier B, Molimard M, Rousselot P, Sitbon O, Simonneau G, Montani D, Humbert
M. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 2016 Sep
1;126(9):3207-18. doi: 10.1172/JCI86249. Epub 2016 Aug 2. PMID: 27482885; PMCID: PMC5004960.
Baumgart B, Guha M, Hennan J, Li J, Woicke J, Simic D, Graziano M, Wallis N, Sanderson T, Bunch RT. In vitro
and in vivo evaluation of dasatinib and imatinib on physiological parameters of pulmonary arterial hypertension.
Cancer Chemother Pharmacol. 2017 Apr;79(4):711-723. doi: 10.1007/s00280-017-3264-2. Epub 2017 Mar 10.
PMID: 28283735.
